Abstract
Gastroesophageal reflux disease (GERD) is a widespread gastrointestinal condition in which acid reflux causes pain, inflammation, and long-term complications. Current treatments provide relief but are often limited by non-specific targeting and systemic side effects. Biopolymer-based carriers offer a promising alternative, and sodium alginate—an abundant, seaweed-derived polysaccharide—forms calcium-crosslinked hydrogels that are biocompatible, low-cost, and
responsive to pH. This study evaluated alginate capsules across pH 2–7 to assess stability and release behavior. Unexpectedly, capsules remained largely intact in strong acid but degraded most extensively at pH 5. This instability highlights a therapeutic window relevant to inflamed esophageal tissue and the gastroesophageal junction. By revealing this overlooked range, the findings suggest alginate’s potential as a simple, scalable platform for targeted delivery not only in GERD but also in other mildly acidic environments such as wound healing and cancer microenvironments.
Keywords: Gastroesophageal reflux disease, alginate, pH-sensitive drug delivery, controlled release
Introduction
Gastroesophageal reflux disease (GERD) affects an estimated 20% of adults in the United States and millions more worldwide, making it a significant public health concern (Katz, Gerson, & Vela, 2013). GERD arises when acidic gastric contents reflux into the esophagus, producing heartburn and regurgitation, and in severe cases, erosive esophagitis and long-term complications. While therapies exist to manage symptoms, their use is often limited by delayed onset, systemic absorption, and adverse effects with prolonged treatment (Hee, Heo, & Lee, 2016). These challenges highlight the need for localized delivery strategies that act directly at the affected site while minimizing unnecessary impact on the rest of the body. This project was motivated by observing the limitations of existing options in a personal context, raising the question of whether treatment could be delivered directly where it is most needed. Alginate, a naturally derived polysaccharide from seaweed, is particularly promising. It forms hydrogels through ionic crosslinking with calcium ions, is generally recognized as safe, and is already widely used in biomedical, food, and engineering applications (Lee & Mooney, 2012).
Previous research has largely focused on alginate’s stability in the stomach’s strongly acidic environment (pH ~2) or the intestine’s near-neutral environment (pH ≥7). However, less attention has been given to intermediate acidity (pH ~4–6), which is highly relevant to inflamed tissues and the gastroesophageal junction. Addressing this gap provides an opportunity to design formulations that release at the precise location where GERD pathology manifests. This paper reviews the literature on alginate hydrogels and their pH-responsive mechanisms, with a focus on their broader biomedical relevance, and presents a student-led case study examining capsule behavior across a pH range of 2–7. Together, this work explores how alginate can be advanced as an accessible, scalable platform for site-specific therapy.
Alginate Hydrogels as Tunable Carriers
Sodium alginate is a naturally occurring, linear polysaccharide extracted from brown algae such as Laminaria spp. and Ascophyllum spp. It consists of two uronic acid monomers—β-D-mannuronic acid (M) and α-L-guluronic acid (G)—arranged in blocks of M, G, or alternating MG sequences. The ratio of M to G strongly influences gel properties: high-G alginates form more rigid, brittle gels, whereas high-M alginates form softer, more elastic networks (Lee & Mooney, 2012).
Gelation occurs when alginate interacts with divalent cations such as calcium (Ca²⁺), which preferentially bind to G-blocks. This results in cooperative junction zones described by the “egg-box” model (Figure 1) stabilizing the polymer into a three-dimensional hydrogel network (Draget & Smidsrød, 2005). The ability to vary M/G ratio and crosslinking conditions makes alginate highly tunable—an essential quality for controlled drug release systems.

Alginate as a Biopolymer
Alginate is also biocompatible, biodegradable, and non-toxic, and it has been recognized as safe for biomedical and food use. These properties, combined with its low cost and global availability, explain why alginate is applied across fields including pharmaceuticals, tissue engineering, and wound dressings (Hariyadi & Islam, 2020; Pawar & Edgar, 2012). Its adaptability means it can be engineered to balance strength, elasticity, and dissolution depending on the therapeutic need.
Controlled Release and Delivery Mechanisms
Alginate hydrogels release encapsulated compounds primarily through diffusion, swelling, and erosion, with the dominant pathway determined by formulation parameters such as alginate concentration, calcium chloride concentration, bead size, and crosslinking time (Ahmad, Pandey, Sharma, & Khuller, 2006). Lightly crosslinked gels allow faster diffusion due to increased porosity, while highly crosslinked gels restrict transport but increase structural stability.
Environmental pH plays a decisive role in alginate behavior. At very low pH (~2), protonation of carboxyl groups reduces electrostatic repulsion and compacts the network, slowing release (Wang, Zhu, Xie, & Wu, 2018). At neutral to basic pH (≥7), calcium ions can be displaced by phosphate or citrate, weakening junctions and promoting erosion (Draget & Smidsrød, 2005). At intermediate pH (~4–6), partial deprotonation coupled with ion exchange destabilizes crosslinks without compaction, often producing greater instability. This nuance explains why alginate does not always degrade most at gastric pH ~2. Instead, mildly acidic environments may represent an underexplored therapeutic window, which is particularly relevant to inflamed tissues in GERD and other pathological conditions.
Alginate in Gastrointestinal Drug Delivery
Alginate carriers have been widely studied for oral applications. For example, alginate nanoparticles encapsulating isoniazid enhanced the bioavailability and stability of isoniazid in tuberculosis treatment (Ahmad, Pandey, & Khuller, 2006). In gastroenterology, alginate beads protect drugs or nutraceuticals from gastric degradation and enable sustained release in distal regions of the gut (Sachan, Pushkar, Jha, & Bhattacharya, 2009). Natural compounds such as curcumin, ginger, and licorice—recognized for anti-inflammatory and gastroprotective effects—have also been encapsulated in alginate systems. Encapsulation improves stability and solubility, particularly for compounds like curcumin that otherwise suffer from poor bioavailability (Gupta, Patchva, & Aggarwal, 2013; Goh, Heng, & Chan, 2012). These studies align with growing interest in safe, food-grade therapies for chronic gastrointestinal conditions. Beyond GERD, alginate’s pH-responsiveness makes it attractive for other biomedical uses. Wound healing environments often have acidic pH values, and alginate dressings have been explored as scaffolds for controlled release of antimicrobials and growth factors (Boateng & Catanzano, 2015). Similarly, many tumors exhibit acidic microenvironments (pH ~6.5), suggesting opportunities for alginate-based systems in cancer-targeted delivery (Liu, Sun, Zhao, & Tang, 2024). This broader relevance highlights alginate’s versatility as a platform technology.
Innovations in Alginate-Based Systems
Recent research has advanced alginate beyond simple gel beads into precision-engineered carriers. Effervescence-assisted systems incorporate carbonate salts that generate CO₂ under acidic conditions, creating internal pressure that ruptures gels and accelerates release (Chia, 2022). Hybrid systems coat alginate with chitosan or enteric polymers to improve selectivity and prevent premature release (Lee & Mooney, 2012). Nanoparticle formulations increase loading capacity, enable the release of hydrophobic compounds, and improve stability against environmental stressors (Wang, Zhang, Li, & Chen, 2022). These innovations demonstrate alginate’s adaptability: it can serve as a simple food-grade bead in resource-limited contexts, or as part of advanced, multi-layered systems in high-tech biomedical research. The literature consistently shows that alginate’s tunability and safety make it one of the most promising natural polymers for controlled release. Importantly, these advances set the stage for the present case study. By investigating capsule behavior in intermediate acidity (pH 5), this project builds on established knowledge while highlighting an underexplored therapeutic niche.
Methods
Capsule Preparation
A 2% (w/v) sodium alginate solution was prepared in deionized water under constant stirring until homogeneous. Capsules were prepared in three exploratory variations:
- Plain alginate capsules – The alginate solution was dispensed dropwise through a 1 mL syringe into a 1.5% (w/v) calcium chloride bath, producing spherical hydrogel beads via ionic crosslinking. Capsules were crosslinked for 1 minute and 30 seconds before being rinsed briefly in distilled water. This duration was identified as optimal for structural stability, as shorter times produced fragile capsules while longer times produced overly rigid beads.
- Carbonate-containing capsules – Sodium bicarbonate (0.06–0.70% w/v) was incorporated into the alginate phase before crosslinking. These capsules exhibited internal clearing and bubbling upon exposure to acidic environments, reflecting effervescence-assisted interactions.
- Payload-containing capsules –In later trials, herbal model compounds were incorporated to test payload release under gastrointestinal conditions. Turmeric, deglycyrrhizinated licorice (DGL), and ginger were selected because they are widely recognized natural anti-inflammatories with reported gastroprotective properties, making them relevant candidates for future therapeutic applications. Each compound was suspended at 1 g in 100 mL of deionized water, and 6 mL of the suspension was blended into 40 mL of 2% alginate solution before crosslinking. These payloads functioned as visible markers of release, providing proof of concept for therapeutic incorporation, though their differing solubility and scattering properties introduced variability in optical measurements.
Testing Procedure
Individual capsules were placed into 50 mL of fixed commercial buffer solutions spanning pH 2–7, contained in 100 mL beakers at room temperature (22–24 °C). Capsule integrity was observed at 5, 10, 15, and 30 minutes, and following overnight exposure. Recorded observations included turbidity, surface softening, internal clearing, rupture, and diffusion of encapsulated material.
Trials and Optimization
Formulations were optimized by varying alginate concentration (1.5–2.5% w/v), calcium chloride concentration (1.0–2.0% w/v), sodium bicarbonate loading (0.0–0.7% w/v), and crosslinking time (1–2 minutes). The combination of 2% sodium alginate, 1.5% calcium
chloride, and 0.7% sodium bicarbonate with a 90-second crosslinking time produced the most consistent behavior, yielding capsules that were stable yet reproducibly degraded at pH 5.
Spectrophotometric Analysis
To complement visual observations, absorbance was measured at 380 nm using a Vernier SpectroVis spectrophotometer. Baseline absorbance of each buffer solution was recorded before capsule addition, and post-exposure absorbance was measured after 30 minutes. The qualitative endpoint was defined as the change in absorbance, where higher values were interpreted as greater release into solution. For each pH condition, baseline absorbance of the buffer solution was measured before capsule addition. After 30 minutes of incubation, post-exposure absorbance was recorded. The qualitative endpoint was defined as the change in absorbance, where higher values were interpreted as greater release of encapsulated material into solution.
Experimental Replicates
Each pH condition was tested in duplicate or triplicate. As this was an exploratory study, the goal was to identify reproducible patterns of capsule behavior rather than to generate statistically validated datasets. While some variability was noted between replicates, the overall trend of greatest breakdown at pH 5 was consistent.
Results
Plain alginate capsules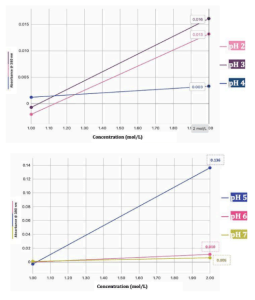
Across the pH range of 2–7, plain alginate capsules displayed distinct differences in breakdown behavior, with the most significant instability observed at pH 5. Figure 2a illustrates this trend clearly: capsules exposed to pH 5 buffers produced an absorbance increase of 0.136 at 380 nm after 30 minutes, far exceeding the responses at pH 6 (0.010) and pH 7 (0.006). By contrast, Figure 2b shows that capsules immersed in strongly acidic conditions (pH 2–4) produced only minor absorbance changes of 0.016 (pH 2), 0.013 (pH 3), and 0.003 (pH 4). These quantitative results aligned with visual inspection. Capsules in pH 2 retained their spherical form with only slight surface softening, consistent with the well-established compaction of alginate gels under strong protonation (Wang, Zhu, Xie, & Wu, 2018). At pH 5, however, erosion began within 10–15 minutes, progressing to near-total collapse by the 30-minute mark. By contrast, capsules at pH 7 showed minimal visible change, remaining stable even overnight.
Taken together, these findings support the conclusion that plain alginate capsules exhibit their greatest instability under mildly acidic conditions. This result is consistent with theoretical expectations: at very low pH, protonation reduces electrostatic repulsion and stabilizes the gel, whereas at intermediate pH, partial deprotonation and calcium ion exchange weaken junction zones, destabilizing the egg-box network (Draget & Smidsrød, 2005; Pawar & Edgar, 2012). Identifying pH 5 as a degradation point highlights an overlooked therapeutic window with practical implications for localized delivery in inflamed esophageal tissue.
Carbonate-containing capsules
When sodium bicarbonate was incorporated into the alginate phase before crosslinking, capsules exhibited distinct effervescence behavior. Within the first 10 minutes of immersion in acidic buffers, bubbling and internal clearing were observed, reflecting CO₂ generation from the reaction of bicarbonate with hydrogen ions. This activity was most pronounced at pH 2, where vigorous but transient bubbling occurred, but at pH 5 the process was slower and more sustained, sometimes leading to partial rupture after overnight incubation.
Spectrophotometric readings supported these observations. Carbonate-containing capsules exposed to pH 5 consistently showed greater increases in absorbance than at pH 2, indicating that while effervescence accelerated local weakening, complete disintegration was more likely in mildly acidic conditions. These results parallel effervescence-assisted release strategies reported in recent literature, where carbonate salts are embedded to promote internal rupture of alginate gels under acid exposure (Chia, 2022; Liu, Sun, Zhao, & Tang, 2024).
Although reproducibility was limited in this student-led study—effervescence varied depending on precise carbonate loading and bead uniformity—the results suggest a promising design principle. Gas-generating additives could be harnessed to trigger capsule disintegration more reliably at clinically relevant sites of pathology, enhancing the release of therapeutic payloads while retaining stability in other regions of the gastrointestinal tract.
Payload-containing capsules
Payload-containing formulations incorporated turmeric, deglycyrrhizinated licorice (DGL), and ginger as model compounds. These natural agents were selected for their widely recognized anti-inflammatory and gastroprotective properties and served as both proof-of-concept payloads and visible markers of release. Visual evidence confirmed release under certain conditions: turmeric-loaded capsules, for instance, showed yellow coloration diffusing into solution at pH 5, while licorice and ginger produced less intense but still observable dispersal in mildly acidic buffers. However, reproducibility across trials was variable. In some runs, payloads remained trapped in intact capsules, while in others, release occurred within the 30-minute observation window.
Several factors likely contributed to this variability: differences in solubility (curcumin is notoriously hydrophobic), light scattering effects from particulate herbal powders, and challenges associated with maintaining consistent buffer volumes across multiple simultaneous trials. Spectrophotometric analysis highlighted these inconsistencies. Although absorbance trends generally indicated greater release at pH 5 compared with pH 2 or 7, irregular baseline shifts complicated interpretation. These limitations underscore the need for standardized dyes or purified model compounds in future work, as complex herbal mixtures introduce uncontrolled variability.
Despite these limitations, the payload trials provide important insight. They demonstrate that alginate matrices are capable of incorporating and releasing biologically relevant compounds under mildly acidic conditions, but they also reveal the experimental rigor required to move from proof-of-concept to reproducible, clinically translatable systems. In the context of existing literature on natural compound encapsulation (Gupta, Patchva, & Aggarwal, 2013; Goh, Heng, & Chan, 2012), these findings reinforce both the promise and the challenges of integrating phytochemicals into alginate delivery platforms.
Conclusion
This study demonstrated that alginate capsules degrade most extensively at pH 5, rather than under stronger gastric acidity. While alginate’s pH sensitivity is well documented, prior research has emphasized behavior at gastric pH (~2) or intestinal pH (≥7). By focusing on the intermediate acidic range, this project highlights a less-studied but clinically important window that aligns with inflamed tissue in GERD. Exploratory carbonate-assisted and payload-loaded trials reinforced this trend, showing how formulation choices can alter release dynamics. Carbonate inclusion generated effervescence that promoted weakening under acid, while herbal payloads provided proof-of-concept evidence of therapeutic incorporation despite variability. These observations underscore alginate’s adaptability and identify design parameters that merit further optimization.
The broader implication is that alginate should not be viewed only as a stomach-protective or colon-targeting material, but as a tunable platform for controlled release in mildly acidic environments. Such conditions are relevant not only to gastroesophageal pathology but also to chronic wounds, infection sites, and tumor microenvironments. Because the material is inexpensive, biocompatible, and globally accessible, these findings carry significance for both advanced biomedical research and low-resource healthcare settings. This work underscores how undergraduate inquiry can uncover underexplored therapeutic opportunities and contribute to the advancement of accessible biomaterials for site-specific therapy. Future studies will incorporate standardized dyes and purified compounds as payloads to improve reproducibility and more accurately model therapeutic release in clinically relevant conditions, as well as experiment with additional formulations and explore computational simulations to guide capsule design before laboratory validation.
